Refractive Index of Polymers
The refractive index (n) is an important optical property of polymers and is widely used in material science. The knowledge of the refractive index is crucial in all optical applications of transparent polymers. Since it is characteristic for each material, it can be used for identification purposes or for the prediction of other properties. For example, the refractive index undergoes a second-order transition at the glass transition temperature, and thus can be used to determine its value. The specific refractive index increment (dn/dc) of dilute polymer solutions, measured in light scattering experiments, is needed in the determination of the molecular weight, and the size and shape of polymers in solutions.
The refractive index is defined as the velocity of light in vacuum relative to the velocity in the polymer. It is directly related to the polarizability and depends on the wavelength of light. It is usually reported for the the yellow sodium doublet line, with a weighted mean of 589.26 nm and symbolized by the letter D.
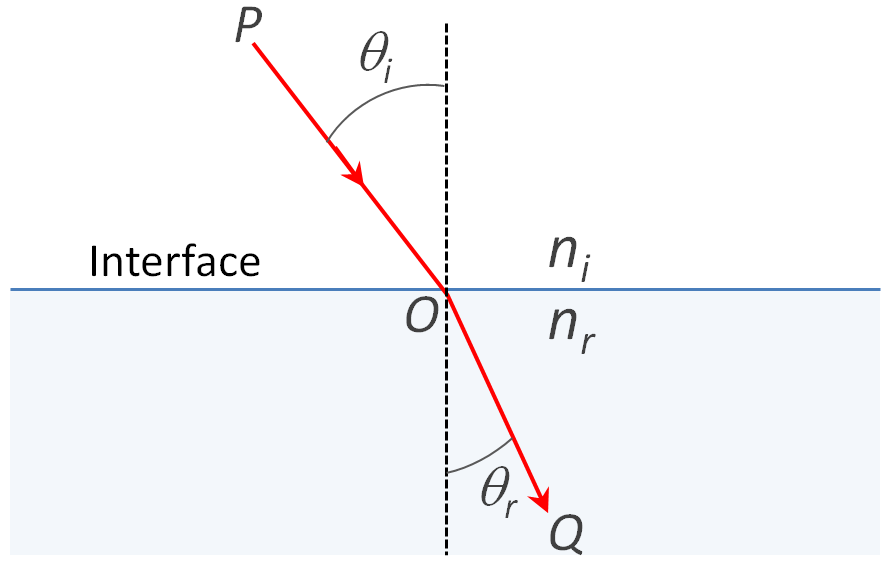
The first law of optical refraction was discoverd by Snellius (1618) and (independently) by Descartes (1637). For light passing from air into a denser medium it is given as
n = sin(θi) / sin(θr)
where n is the index of refraction; and θi and θr are the angles of incident and refracted light. A similar law can be applied to light that crosses frome one material into another material:
ni / nr = sin(θi) / sin(θr)
where ni and nr are the refractive index of the incident and refractive medium.
In 1858 Gladstone and Dale observed that the ratio (n - 1) / ρ is a characteristic constant for all organic substances which is called the specific refraction. They also found that the product of the specific refraction and the molar mass (M) has addititive properties in regard to the structural groups in the molecule:
M (n - 1) / ρ = V (n - 1) = RGD = ∑i RGD,i
This property is called molar refraction,
R. The quantity (n - 1) is sometimes called the “brake
power” of the material and the ratio (n – 1)/ρ the
specific relative brake power.
Another expression for the molar refraction was developed by Lorentz and Lorenz (1880), which has also additivite properties:
M/ρ · (n2 - 1) / (n2 + 2) = V (n2 - 1) / (n2 + 2) = RLL = ∑i RLL,i
Finally, in the 1950s, Vogel suggested a simpler expression that is only valid at constant temperature:
M / n = RV = ∑i RV,i
The three molar refractions, RV, RGD and RLL, can be calculated as a sum of corresponding atom, bond or group contributions, and the molar volume can be either estimated as a van der Waals volume of the compound divided by the average coefficient of molecular packing or it can be directly calculated as a sum of corresponding atom or group contributions.
The main advantage of using contributions methods are their
simplicity. The prediction are reasonable accurate provided all the necessary increments are known from the experimental data for every
structural element. However, in certain cases, interactions between functional groups can cause noticeable errors in the predicted refractive index values. For example, the predicted refractive
indices of
compounds with π-conjugated groups can noticebly deviate from the true refractive indices. In some cases, the deviation from the experimental values can be more than 20 percent. The source of these
discrepancies is believed to be large optical dispersion, for
example by π-electron delocalization effects in conjugated polymers.
Chemical bond and group methods for the prediction of refractive indices of (amorphous) polymers have been summarized by Van Krevelen1 and by Blythe2. A (semiempirical) atom contribution method based on the Lorenz–Lorentz formula has been developed by Askadskii.3
Refractive Indices of Polymer Blends
For heterogenous polymers and polymer blends with phase dimensions below the wavelength of the incident light, the application of the Maxwell relations is valid and the upper bound of the refractive index of the copolymer can be obtained from the sum of the dielectric constants of the phases, ε = n2:
n2 = ∑i νi ni2
where ni and νi are the refractive indices and volume fractions of the constituent phases.
The lower bound of the refractive index of heterogeous polymers may be calculated as the sum of the inverse of the dielectric constants of the constituent phases:
n-2 = ∑i νi / ni2
If the refractive indices differ by less than 0.2, the refractive index of the heteropolymer may be predicted by a simple additivity approach based on the volume fractions:
n =∑i νi ni
This equation can be applied to blends, block or graft copolymers, composites, and semi-crystalline polymers.
In the case of a semi-crystalline polymer, the refractive index of the blend is given by
n = νcr nr + (1 - νcr) nam
where νcr is the volume fraction of the crystalline portion, which can be calculated from the densities of the amorphous and crystalline phases:
νcr = (ρ - ρam) / (ρcr - ρam)
where ρ is the (measured) bulk density, and ρcr and ρam are the crystalline and noncrystalline densities.
References & Notes
-
D.W. van Krevelen and Klaas te Nijenhuis, Properties of Polymers, 4th Ed. Amsterdam (2009)
-
A. R. Blythe, Electrical Properties of Polymers, Cambridge University Press 1979.
-
Andrey A. Askadskii, Computational Material Science of Polymers, Cambridge 2003